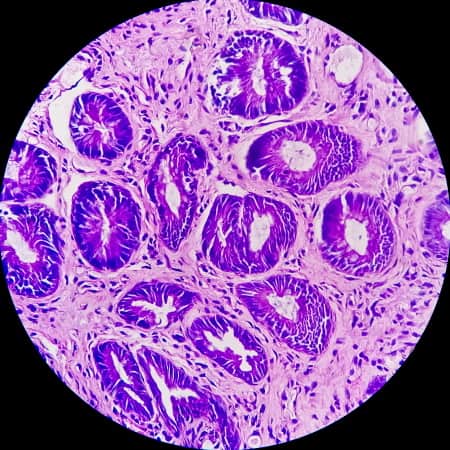
The study, which was sponsored by the drug company GlaxoSmithKline, was presented Sunday at the annual meeting of the American Society of Clinical Oncology. The study authors reported several surprises: None of the patients needed other treatments associated with rectal cancer, such as life-altering surgery or chemotherapy, and none had clinically significant complications.
“There were a lot of happy tears,” Andrea Cercek, MD, the lead study author and an oncologist at Memorial Sloan Kettering Cancer Center, told the newspaper.
But the study was small, she noted, and it’ll take time to understand whether the patients will remain in remission.
Republished with permission[/vc_message]












