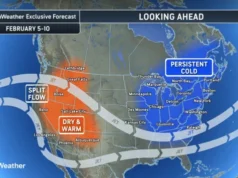
The FDA’s sudden shift highlights challenges regulators and the pharmaceutical industry have faced amid unprecedented demand for the blockbuster weight-loss drugs.
“The credibility of the FDA in this case has suffered,” said Mariana Socal, a Johns Hopkins professor who specializes in drug shortages. She attributed the agency’s flip-flop to its surveillance system, which by law relies heavily on information from drugmakers and is geared toward identifying disruptions to supply rather than spikes in demand.
“That system is not necessarily in real time reflecting the correct balance of the market,” she said.
The recent upheaval began Oct. 2, when the FDA declared the Mounjaro and Zepbound shortage over after almost two years. It provided little detail other than saying that Eli Lilly had shown it could “meet the present and projected national demand,” while acknowledging that patients could still see periodic shortages due in part to the logistics of shipping the refrigerated medication.
Disclaimer
The information contained in South Florida Reporter is for general information purposes only.
The South Florida Reporter assumes no responsibility for errors or omissions in the contents of the Service.
In no event shall the South Florida Reporter be liable for any special, direct, indirect, consequential, or incidental damages or any damages whatsoever, whether in an action of contract, negligence or other tort, arising out of or in connection with the use of the Service or the contents of the Service.
The Company reserves the right to make additions, deletions, or modifications to the contents of the Service at any time without prior notice.
The Company does not warrant that the Service is free of viruses or other harmful components