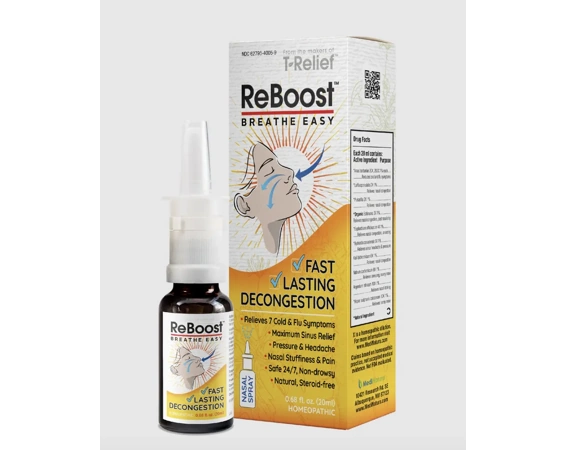
A nationwide recall is underway for a nasal spray due to bacterial contamination, the Food and Drug Administration (FDA) announced Wednesday. One lot of ReBoost Nasal Spray was found to contain yeast/mold and microbials, including unsafe levels of the bacteria Achromobacter. The product could cause adverse health consequences—including life-threatening infections—in people with weakened immune systems. To date, there have been no reports of illness related to the product.
How to Identify the Recalled Nasal Spray
The recalled nasal spray, produced by MediNatura New Mexico Inc., can be identified by the following information:
- Product name: ReBoost Nasal Spray
- Size: 0.68 fl oz (20 mL)
- NDC: 62795-4005-9
- UPC: 787647 10186 3
- Lot number: 224268
- Expiration date: 12/2027
The recalled product was sold nationwide at retailers and online through the MediNatura website.
:max_bytes(150000):strip_icc():format(webp)/recalled-nasal-spray-4bd5060a8b9f4110b941b003061cefd0.png)
What You Should Do
Check your medicine cabinet for the recalled nasal spray. If you have it, do not use it—return it to the store for a refund. If you purchased it directly from the MediNatura website, email recall@medinatura.com to arrange for a refund.
If you think you’ve used a contaminated nasal spray and start to experience health problems, contact a healthcare provider. You can also report adverse events related to the recalled product to the FDA’s MedWatch Adverse Event Reporting program.
Disclaimer
Artificial Intelligence Disclosure & Legal Disclaimer
AI Content Policy.
To provide our readers with timely and comprehensive coverage, South Florida Reporter uses artificial intelligence (AI) to assist in producing certain articles and visual content.
Articles: AI may be used to assist in research, structural drafting, or data analysis. All AI-assisted text is reviewed and edited by our team to ensure accuracy and adherence to our editorial standards.
Images: Any imagery generated or significantly altered by AI is clearly marked with a disclaimer or watermark to distinguish it from traditional photography or editorial illustrations.
General Disclaimer
The information contained in South Florida Reporter is for general information purposes only.
South Florida Reporter assumes no responsibility for errors or omissions in the contents of the Service. In no event shall South Florida Reporter be liable for any special, direct, indirect, consequential, or incidental damages or any damages whatsoever, whether in an action of contract, negligence or other tort, arising out of or in connection with the use of the Service or the contents of the Service.
The Company reserves the right to make additions, deletions, or modifications to the contents of the Service at any time without prior notice. The Company does not warrant that the Service is free of viruses or other harmful components.