“Today we are announcing that 14 companies peddling bogus cancer cures have received warning letters,” write physicians at the US Food and Drug Administration (FDA) in a blog post.
“A cancer diagnosis often provokes a sense of desperation,” they write. “Unfortunately, rogue operations exploiting those fears peddle untested and potentially dangerous products, particularly on the internet.”
The FDA is now taking the first step to stamp out such practices. The warning letters are a primary compliance tool that the FDA uses to address violations of the Federal Food, Drug and Cosmetic Act, the authors explain.
At the same time, the agency is publicizing its actions against the bogus products with warnings sent to consumers.
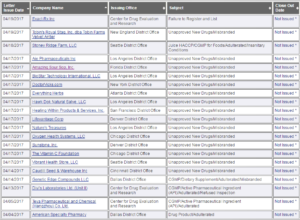
The warning letters were sent to 14 US-based companies illegally selling more than 65 products that fraudulently claim to prevent, diagnose, treat, or cure cancer.
Most commonly sold on websites and social media platforms, these products are being marketed with “slick ads, videos, and other sophisticated marketing techniques, including testimonials about miraculous outcomes,” the FDA officials comment.
The authors are Donald D. Ashley, MD, director of the Office of Compliance in FDA’s Center for Drug Evaluation and Research, and Douglas Stearn, MD, director of the Office of Enforcement and Import Operations in FDA’s Office of Regulatory Affairs.